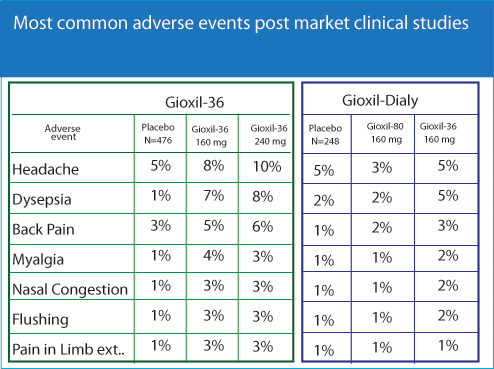
GIOXIL-36 was generally well tolerated in clinical trials*
Cardiovascular safety
Pooled results from 36 controlled and open-label clinical trials are presented in the table below. Doses of GIOXIL-36 ranged from 2 mg to 50 mg, with most patients receiving 20 mg. Patients were excluded from trials if they had an underlying cardiovascular disorder sufficiently severe or unstable to make sexual intercourse inadvisable.
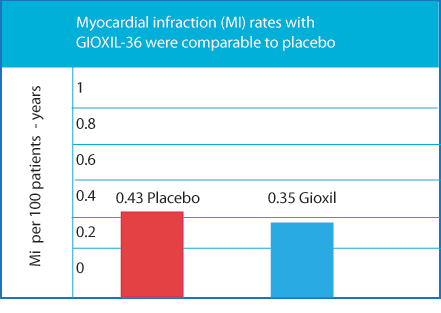
GIOXIL-36 taken as needed n=12,978; GIOXIL-36 taken once daily n=10772
The incidence of serious cardiovascular treatment-emergent adverse events with GIOXIL-36 taken once daily was comparable to placebo§2
One patient with comorbid hypertension and hyperlipidemia who received GIOXIL-36 2.5 mg for once daily use had a nonfatal MI3 |
